pH is one of the most important and simple metrics to monitor throughout the brewing process. This method details how to carry out measurements and calibrations using a digital pH meter to ensure accurate results.

Figure 1 - Click to englarge
Digital pH meters consist of two parts; a probe and the meter. The probe is what will be submersed into the sample and the meter is the computer that will display the results. These can either be two separate parts connected by a cord (see Figure 1) or contained all in one.

Figure 2 - Click to englarge
When performing a calibration, the computer within the meter creates a calibration curve where pH is the x-axis variable and voltage is the y-axis variable, or the response (see Figure 2). Depending on the meter, calibration curves can either be a one, two, or three point calibration using 4.0, 7.0, and 10.0 pH buffers. A calibration curve will be more accurate the more points used so if possible calibrate with three points. If limited by the meter, calibrate closest to the range of the samples. For beer this would be a pH of 4. This demonstrates why it is important to use fresh, unexpired buffers. If the buffers are not the values you expect them to be the calibration will be inaccurate and so will the pH results.
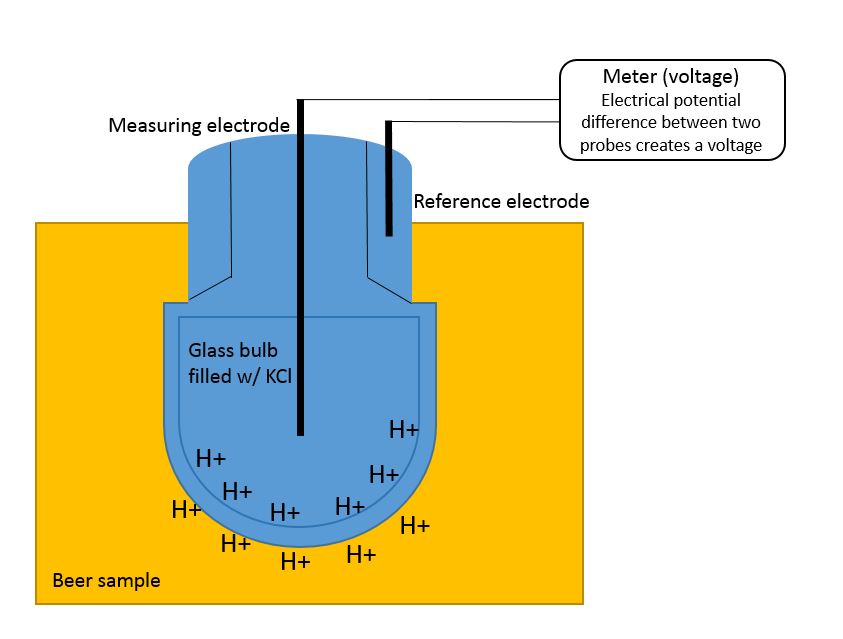
Figure 3, image credit: Katie Fromuth - Click to Enlarge
The glass electrode probe is filled with a potassium chloride (KCl) solution with a neutral pH of 7 and a known amount of hydrogen ions. The storage solution is also a potassium chloride solution to keep the hydrogen ions inside the probe constant. If a test solution is acidic it contains more hydrogen ions than inside the probe. The opposite is true for basic solutions. The hydrogen ions inside and outside the glass electrode will interact and create a difference in voltages between the measuring electrode and reference electrode (see Figure 3). The voltage (mV) response is based off of the Nernst Equation. Using the calibration curve, the meter will compute what pH the test solution is.
Green Chemistry
|
Green Action |
Principle |
Benefit |
| Degas by sonication or agitation |

Prevent Waste
| Eliminates costs and wastes associated with degassing by filter paper. |
| Compost filter paper if use for degassing is necessary. |

Atom Economy | Minimizes filter paper waste to landfill |
Image credit: iconsmind.com
A Big Thanks To...
Ann Sandbrook, Joe Palausky, & Katie Fromuth